Radiocarbon dating
Radiocarbon dating (or simply carbon dating) is a radiometric dating technique that uses the decay of carbon-14 (14C) to estimate the age of organic materials, such as wood and leather, up to about 58,000 to 62,000 years Before Present (BP, present defined as 1950). Carbon dating was presented to the world by Willard Libby in 1949, for which he was awarded the Nobel Prize in Chemistry. Since its introduction it has been used to date many items, including samples of the Dead Sea Scrolls, the Shroud of Turin, enough Egyptian artifacts to supply a chronology of Dynastic Egypt,[2] and Ötzi the Iceman.
The Earth's atmosphere contains various isotopes of carbon, roughly in constant proportions. These include the main stable isotope (12C) and an unstable isotope (14C). Through photosynthesis, plants absorb both forms from carbon dioxide in the atmosphere. When an organism dies, it contains the standard ratio of 14C to 12C, but as the 14C decays with no possibility of replenishment, the proportion of carbon 14 decreases at a known constant rate.
The time taken for it to reduce by half is known as the half-life of 14C. The measurement of the remaining proportion of 14C in organic matter thus gives an estimate of its age (a raw radiocarbon age).[4] However, over time there are small fluctuations in the ratio of 14C to 12C in the atmosphere, fluctuations that have been noted in natural records of the past, such as sequences of tree rings and cave deposits. These records allow fine-tuning, or "calibration", of the raw radiocarbon age, to give a more accurate estimate of the calendar date of the material.
One of the most frequent uses of radiocarbon dating is to estimate the age of organic remains from archaeological sites.
 |
Carbon dating is a variety o fradioactive dating which is applicable only to matter which was once living and presumed to be in equilibrium with the atmosphere, taking in carbon dioxide from the air for photosynthesis.
Cosmic ray protons blast nuclei in the upper atmosphere, producing neutrons which in turn bombard nitrogen, the major constituent of the atmosphere . This neutron bombardment produces the radioactive isotope carbon-14. The radioactive carbon-14 combines with oxygen to form carbon dioxide and is incorporated into the cycle of living things.
The carbon-14 forms at a rate which appears to be constant, so that by measuring the radioactive emissions from once-living matter and comparing its activity with the equilibrium level of living things, a measurement of the time elapsed can be made.
|
Radioactive Dating
Because the radioactive half-life of a given radioisotope is not affected by temperature, physical or chemical state, or any other influence of the environment outside the nucleus save direct particle interactions with the nucleus, then radioactive samples continue to decay at a predictable rate. That is, any radioactive nucleus acts as a clock. If determinations or reasonable estimates of the original composition of a radioactive sample can be made, then the amounts of the radioisotopes present can provide a measurement of the time elapsed.
One such method is called carbon dating, which is limited to the dating of organic (once living) materials. The longer-lived radioisotopes in minerals provide evidence of long time scales in geological processes. While original compositions cannot be determined with certainty, various combination measurements provide self-consistent values for the the times of formations of certain geologic deposits. These clocks-in-the-rocks methods provide data for modeling the formation of the Earth and solar system.
Carbon Dating
Presuming the rate of production of carbon-14 to be constant, the activity of a sample can be directly compared to the equilibrium activity of living matter and the age calculated. Various tests of reliability have confirmed the value of carbon data, and many examples provide an interesting range of application.
Carbon-14 decays with a halflife of about 5730 years by the emission of an electron of energy 0.016 MeV. This changes the atomic number of the nucleus to 7, producing a nucleus of nitrogen-14. At equilibrium with the atmosphere, a gram of carbon shows an activity of about 15 decays per minute.
The low activity of the carbon-14 limits age determinations to the order of 50,000 years by counting techniques. That can be extended to perhaps 100,000 years byaccelerator techniques for counting the carbon-14 concentration.
How Carbon-14 is Made Cosmic rays enter the earth's atmosphere in large numbers every day. For example, every person is hit by about half a million cosmic rays every hour. It is not uncommon for a cosmic ray to collide with an atom in the atmosphere, creating a secondary cosmic ray in the form of an energetic neutron, and for these energetic neutrons to collide with nitrogen atoms. When the neutron collides, a nitrogen-14 (seven protons, seven neutrons) atom turns into a carbon-14 atom (six protons, eight neutrons) and a hydrogen atom (one proton, zero neutrons). Carbon-14 is radioactive, with a half-life of about 5,700 years. For more information on cosmic rays and half-life, as well as the process of radioactive decay, seeHow Nuclear Radiation Works. Carbon-14 in Living Things The carbon-14 atoms that cosmic rays create combine with oxygen to form carbon dioxide, which plants absorb naturally and incorporate into plant fibers by photosynthesis. Animals and people eat plants and take in carbon-14 as well. The ratio of normal carbon (carbon-12) to carbon-14 in the air and in all living things at any given time is nearly constant. Maybe one in a trillion carbon atoms are carbon-14. The carbon-14 atoms are always decaying, but they are being replaced by new carbon-14 atoms at a constant rate. At this moment, your body has a certain percentage of carbon-14 atoms in it, and all living plants and animals have the same percentage. |
Carbon-14 Equilibrium Activity
Since living organisms continually exchange carbon with the atmosphere in the form of carbon dioxide, the ratio of C-14 to C-12 approaches that of the atmosphere.
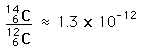
From the known half-life of carbon-14 and the number of carbon atoms in a gram of carbon, you can calculate the number of radioactive decays to be about 15 decays per minute per gram of carbon in a living organism.
Radioactive carbon is being created by this process at the rate of about two atoms per second for every square centimeter of the earth's surface." Levin
The rate of production of carbon-14 in the atmosphere seems to be fairly constant. Carbon dating of ancient bristlecone pine trees of ages around 6000 years have provided general corroboration of carbon dating and have provided some corrections to the data.
Bristlecone Pine Trees
From the dating of ancient bristlecone pine trees from the western U.S., a correction curve for the carbon dating over the range back to 5000 BC has been developed. Trees dated at 4000 BC show the maximum deviation of between 600 and 700 years too young by carbon dating.
Glacier Measurements
Prior to carbon dating methods, the age of sediments deposited by the last ice age was surmised to be about 25000 years. "Radiocarbon dates of a layer of peat beneath the glacial sediments provided an age of only 11,400 years."
On the other hand, atmospheric testing of nuclear weapons in the 1950s and 1960s increased the carbon-14 content of the atmosphere. Krane suggests that this might have doubled the concentration compared to the carbon-14 from cosmic ray production.
Accelerator Techniques for Carbon Dating
Accelerator techniques for carbon dating have extended its range back to about 100,000 years, compared to less than half that for direct counting techniques. One can count atoms of different masses with a mass spectrometer, but that is problematic for carbon dating because of the low concentration of carbon-14 and the existence of nitrogen-14 and CH2 which have essentially the same mass.
Cyclotrons and tandem accelerators have both been used to fashion sensitive new mass spectrometer analyses. The tandem accelerator has been effective in removing the nitrogen-14 and CH2, and can be followed by a conventional mass spectrometer to separate the C-12 and C-13. A sensitivity of 10-15 in the 14C/12C ratio has been achieved. These techniques can be applied with a sample as small as a milligram.
Mass Spectrometer
The mass spectrometer is an instrument which can measure the masses and relative concentrations of atoms and molecules. It makes use of the basic magnetic force on a moving charged particle.
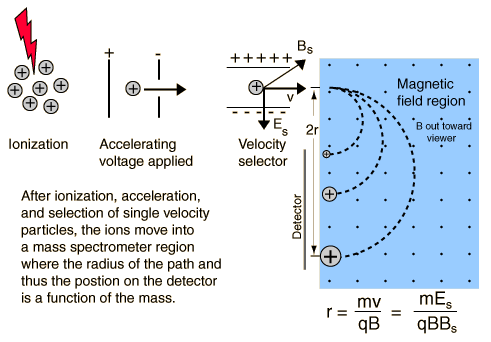
Circular Path from Magnetic Field
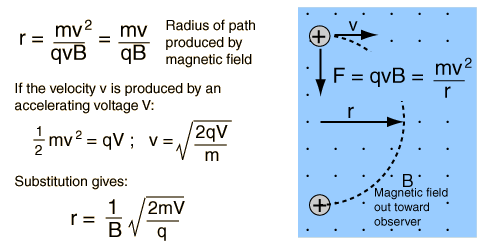
If a charge moves into a magnetic field with direction perpendicular to the field, it will follow a circular path. The magnetic force, being perpendicular to the velocity, provides the centripetal force.
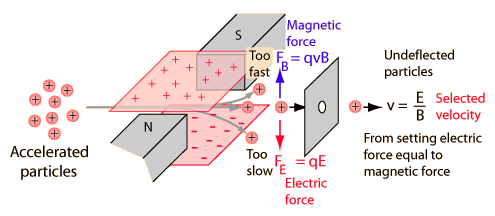
A velocity selector is used with mass spectrometers to select only charged particles with a specific velocity for analysis. It makes use of a geometry where opposing electric and magnetic forces match for a specific particle speed. It therefore lets through undeflected only those particles with the selected velocity.
Mass spectrometers are sensitive detectors of isotopes based on their masses. They are used in carbon dating and other radioactive dating processes. The combination of a mass spectrometer and a gas chromatograph makes a powerful tool for the detection of trace quantities of contaminants or toxins. A number of satellites and spacecraft have mass spectrometers for the identification of the small numbers of particles intercepted in space. For example, the SOHO satellite uses a mass spectrometer to analyze the solar wind.
Mass spectrometers are used for the analysis of residual gases in high vacuum systems.
 |
Mass Spectrometer Analysis
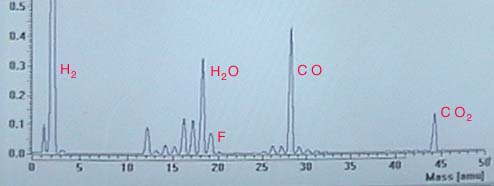
The image below is a mass spectrometer analysis of the residual gases remaining in a high vacuum system after pumping to a pressure around 10-10torr and baking at around 150 C. The water, carbon monoxide and carbon dioxide are commonly the remaining gases in laboratory high vacuum systems. The largest signal was from molecular hydrogen, with a peak height almost three times that of carbon monoxide. The peak at 19 had been puzzling to the experimenters until they had determined that teflon releases some flourine at those temperatures, so the source was apparently the teflon insulation on wires inside the vacuum. The vertical axis shows ion current in 10-7 Amperes.

Magnetohydrodynamics
A magnetohydrodynamic generator has been described as a magnet on the tail of a jet engine. A super-hot plasma is created, ionizing the atoms of the fuel mixture. The magnetic field deflects positive and negative charges in different directions. Collecting plates for the charges provide a DC voltage.
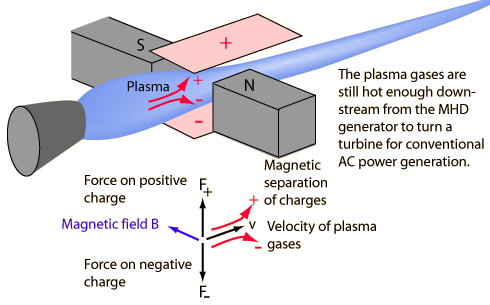
A magnetohydrodynamic generator has been described as a magnet on the tail of a jet engine. A super-hot plasma is created, ionizing the atoms of the fuel mixture. The magnetic field deflects positive and negative charges in different directions. Collecting plates for the charges provide a DC voltage.

Magneto hydrodynamics as an electricity generation process holds the possibility of very efficient fuel utilization because the extremely high temperatures at which it operates correlate to a high Carnot efficiency. Its practical application has been slow in coming because of a number of problems, including a high rate of damage to the combustion chamber by the high velocity particles.
Geiger–Müller counter
A Geiger–Müller counter, also called a Geiger counter, is a type of particle detector that measures ionizing radiation. It detects the emission of nuclear radiation — alpha particles,beta particles, and gamma rays — by the ionization produced in a low-pressure gas in aGeiger–Müller tube,[1] which gives its name to the instrument. In wide and prominent use as a hand-held radiation survey instrument, it is perhaps one of the world's best-known radiation instruments.
The original operating principle was discovered in 1908 in early radiation research.
Since the subsequent development of the Geiger-Müller tube in 1928 the Geiger-Müller counter has been a popular instrument for use in radiation dosimetry, health physics, experimental physics, the nuclear industry, geological exploration and other fields, due to its robust sensing element and relatively low cost.
However there are limitations in measuring high radiation rates and in measuring the energy of incident radiation.
The meter shown is a sensitive survey meter which is used in a research lab to detect low levels of radiation. Geiger counters are ionization detectors which operate at a high voltage, in the region where an avalanche pulse is produced by a single event.
This is a closer view of the scale and the detector head. A thin molybdenum window is often used on the detector heads.
A typical configuration for Geiger counters has been a wire on the centerline of a cylinder. A high voltage is maintained between the wire and the cylinder. When a high energy particle enters the cavity and ionizes a few air molecules, the free electrons are accelerated strongly toward the wire. In the process, those electrons ionize many more air molecules, producing a current pulse. Survey meters like the one shown often convert the current pulses into audible clicks, producing the sound popularly associated with Geiger counters.



Sem comentários:
Enviar um comentário