Radioactivity
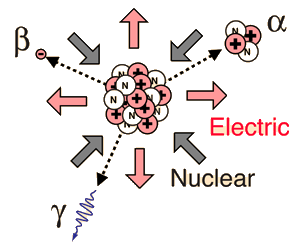 |
Radioactivity refers to the particles which are emitted from nuclei as a result of nuclear instability. Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. The most common types of radiation are called alpha, beta, and gamma radiation, but there are several other varieties of radioactive decay.
|
Radioactive decay rates are normally stated in terms of their half-lives, and the half-life of a given nuclear species is related to its radiation risk. The different types of radioactivity lead to different decay paths which transmute the nuclei into other chemical elements. Examining the amounts of the decay products makes possible radioactive dating.
Radiation from nuclear sources is distributed equally in all directions, obeying the inverse square law.
Alpha Radioactivity
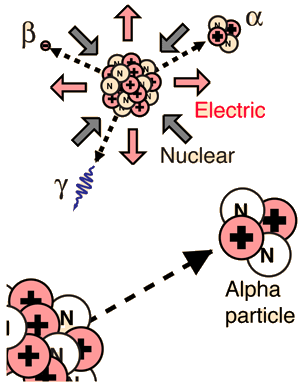 |
Composed of two protons and two neutrons, the alpha particle is a nucleus of the element helium. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it has a very short range. It is not suitable for radiation therapy since its range is less than a tenth of a millimeter inside the body. Its main radiation hazard comes when it is ingested into the body; it has great destructive power within its short range. In contact with fast-growing membranes and living cells, it is positioned for maximum damage.
|
Alpha particle emission is modeled as a barrier penetration process. The alpha particle is the nucleus of the helium atom and is the nucleus of highest
Alpha Binding Energy
The nuclear binding energy of the alpha particle is extremely high, 28.3 MeV. It is an exceptionally stable collection of nucleons, and those heavier nuclei which can be viewed as collections of alpha particles (carbon-12, oxygen-16, etc.) are also exceptionally stable. This contrasts with a binding energy of only 8 MeV for helium-3, which forms an intermediate step in the proton-proton fusion cycle.
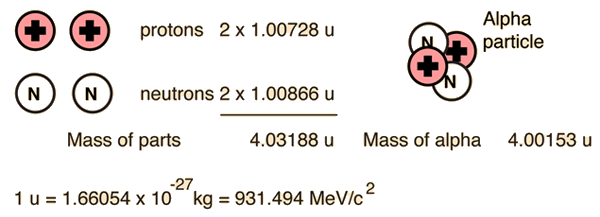
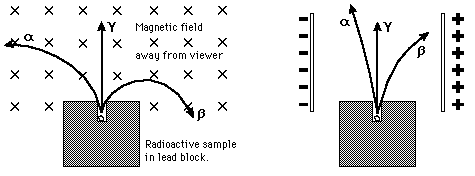
Historically, the products of radioactivity were called alpha, beta, and gamma when it was found that they could be analyzed into three distinct species by either a magnetic field or an electric field.
Penetration of Matter
Though the most massive and most energetic of radioactive emissions, the alpha particle is the shortest in range because of its strong interaction with matter. The electromagnetic gamma ray is extremely penetrating, even penetrating considerable thicknesses of concrete. The electron of beta radioactivity strongly interacts with matter and has a short range.
Radioactivity was discovered by A. H. Becquerel in 1896. The radiation was classified by E. Rutherford as alpha, beta, and gamma rays according to their ability to penetrate matter and ionize air.
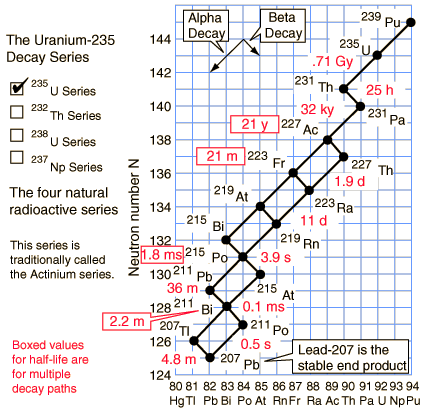
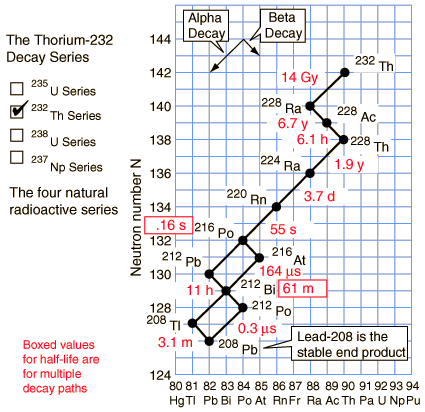
The members of this series are not presently found in nature because the half-life of the longest lived isotope in the series is short compared to the age of the earth.
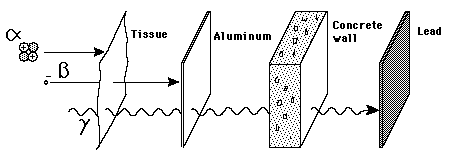 |
The electromagnetic gamma ray is extremely penetrating, even penetrating considerable thicknesses of concrete. |

Sem comentários:
Enviar um comentário