Fast Breeder Reactors
Under appropriate operating conditions, the neutrons given off by fission reactions can "breed" more fuel from otherwise non-fissionable isotopes. The most common breeding reaction is that of plutonium-239 from non-fissionable uranium-238. The term "fast breeder" refers to the types of configurations which can actually produce more fissionable fuel than they use, such as the LMFBR. This scenario is possible because the non-fissionable uranium-238 is 140 times more abundant than the fissionable U-235 and can be efficiently converted into Pu-239 by the neutrons from a fission chain reaction.

France has made the largest implementation of breeder reactors with its large Super-Phenix reactor and an intermediate scale reactor (BN-600) on the Caspian Sea for electric
Plutonium Breeding Ratio
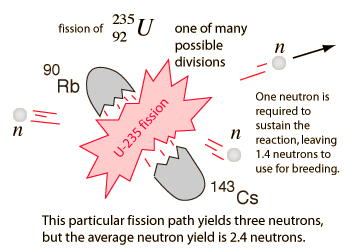
In the breeding of plutonium fuel in breeder reactors, an important concept is the breeding ratio, the amount of fissile plutonium-239 produced compared to the amount of fissionable fuel (like U-235) used to produced it. In the liquid-metal, fast-breeder reactor (LMFBR), the target breeding ratio is 1.4 but the results achieved have been about 1.2 . This is based on 2.4 neutrons produced per U-235 fission, with one neutron used to sustain the reaction.
The time required for a breeder reactor to produce enough material to fuel a second reactor is called its doubling time, and present design plans target about ten years as a doubling time.
A reactor could use the heat of the reaction to produce energy for 10 years, and at the end of that time have enough fuel to fuel another reactor for 10 years.
Liquid-Metal, Fast-Breeder Reactor
The plutonium-239 breeder reactor is commonly called a fast breeder reactor, and the cooling and heat transfer is done by a liquid metal. The metals which can accomplish this are sodium and lithium, with sodium being the most abundant and most commonly used.
The construction of the fast breeder requires a higher enrichment of U-235 than a light-water reactor, typically 15 to 30%. The reactor fuel is surrounded by a "blanket" of non-fissionable U-238. No moderator is used in the breeder reactor since fast neutrons are more efficient in transmuting U-238 to Pu-239. At this concentration of U-235, the cross-section for fission with fast neutrons is sufficient to sustain the chain-reaction. Using water as coolant would slow down the neutrons, but the use of liquid sodium avoids that moderation and provides a very efficient heat transfer medium.
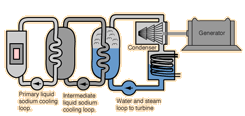 Illustration | Cool with liquid sodium? Isn't that stuff dangerous? |
Liquid-Metal Fast-Breeder Reactor
In the LMFBR, the fission reaction produces heat to run the turbine while at the same time breeding plutonium fuel for the reactor.
 |
| Adicionar legenda |
Boiling Water Reactor
In the boiling water reactor the same water loop serves as moderator, coolant for the core, and steam source for the turbine.
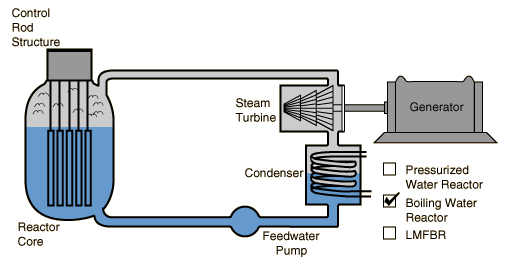
Boiling Water Reactor
In the boiling water reactor (BWR), the water which passes over the reactor core to act as moderator and coolant is also the steam source for the turbine. The disadvantage of this is that any fuel leak might make the water radioactive and that radioactivity would reach the turbine and the rest of the loop.
A typical operating pressure for such reactors is about 70 atmospheres at which pressure the water boils at about 285¡C. This operating temperature gives a Carnot efficiency of only 42% with a practical operating efficiency of around 32%, somewhat less than the PWR.
Pressurized Water Reactor
In the pressurized water reactor, the water which flows through the reactor core is isolated from the turbine.
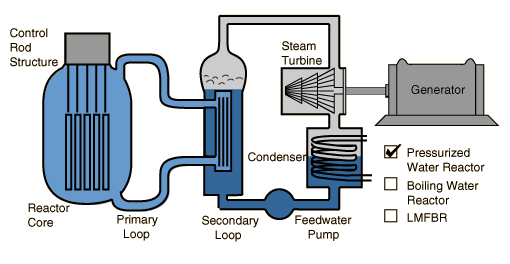
Pressurized Water Reactors
In the pressurized water reactor (PWR), the water which passes over the reactor core to act as moderator and coolant does not flow to the turbine, but is contained in a pressurized primary loop. The primary loop water produces steam in the secondary loop which drives the turbine. The obvious advantage to this is that a fuel leak in the core would not pass any radioactive contaminants to the turbine and condenser.
Another advantage is that the PWR can operate at higher pressure and temperature, about 160 atmospheres and about 315 C. This provides a higher Carnot efficiency than the BWR, but the reactor is more complicated and more costly to construct. Most of the U.S. reactors are pressurized water reactors.
Liquid-Metal Fast-Breeder Reactor
In the LMFBR, the fission reaction produces heat to run the turbine while at the same time breeding plutonium fuel for the reactor.

Liquid-Metal, Fast-Breeder Reactor
The plutonium-239 breeder reactor is commonly called a fast breeder reactor, and the cooling and heat transfer is done by a liquid metal. The metals which can accomplish this are sodium and lithium, with sodium being the most abundant and most commonly used.
The construction of the fast breeder requires a higher enrichment of U-235 than a light-water reactor, typically 15 to 30%. The reactor fuel is surrounded by a "blanket" of non-fissionable U-238. No moderator is used in the breeder reactor since fast neutrons are more efficient in transmuting U-238 to Pu-239.
At this concentration of U-235, the cross-section for fission with fast neutrons is sufficient to sustain the chain-reaction. Using water as coolant would slow down the neutrons, but the use of liquid sodium avoids that moderation and provides a very efficient heat transfer medium.
 Illustration |
Liquid Sodium Coolant
Liquid sodium is used as the coolant and heat-transfer medium in the LMFBR reactor. That immediately raised the question of safety since sodium metal is an extremely reactive chemical and burns on contact with air or water (sometimes explosively on contact with water). It is true that the liquid sodium must be protected from contact with air or water at all times, kept in a sealed system. However, it has been found that the safety issues are not significantly greater than those with high-pressure water and steam in the light-water reactors.
Sodium is a solid at room temperature but liquifies at 98°C. It has a wide working temperature since it does not boil until 892°C. That brackets the range of operating temperatures for the reactor so that it does not need to be pressurized as does a water-steam coolant system. It has a large specific heatso that it is an efficient heat-transfer fluid.
In practice, those reactors which have used liquid metal coolants have been fast-neutron reactors. The liquid metal coolant has a major advantage there because water as a coolant also moderates or slows down the neutrons. Such fast-neutron reactors require a higher degree of enrichment of the uranium fuel than do the water moderated reactors.
Physical Properties of Some Typical Liquids
| Density (kg/m3) | ||||
| Latent heat of fusion (kJ/mol) | ||||
| Latent heat of evaporation (kJ/mol) | ||||
| Heat capacity (J/(mol K) | ||||
| Melting point (K) | ||||
| Liquid range (K) | ||||
| Isothermal compressibility (1/(N m2)) | ||||
| Surface tension(mJ/ m2) | ||||
| Viscosity (Poise=0.1kg/(m s)) | ||||
| Self-diffusion coefficient (m2/s) | ||||
| Thermal conductivity (J/(m s K)) | ||||
| Density (kg/m3) | ||||
| Latent heat of fusion (kJ/mol) | ||||
| Latent heat of evaporation (kJ/mol) | ||||
| Heat capacity (J/(mol K) | ||||
| Melting point (K) | ||||
| Liquid range (K) | ||||
| Isothermal compressibility (10-10 N-1 m2) | ||||
| Surface tension(mJ/ m2) | ||||
| Viscosity (Poise=0.1kg/(m s)) | ||||
| Self-diffusion coefficient (m2/s) | ||||
| Thermal conductivity (J/(m s K)) | ||||
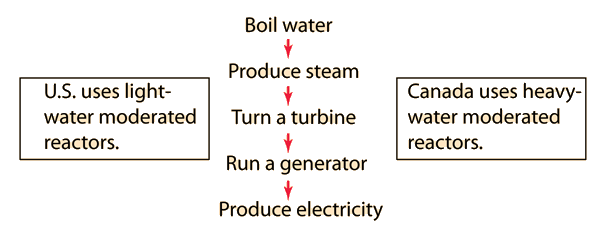
Light Water Reactors
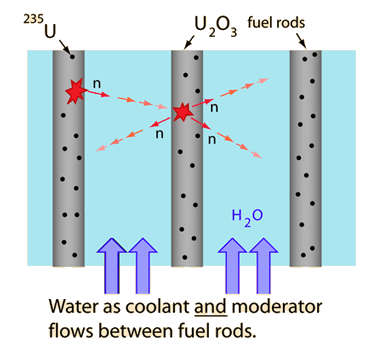
The nuclear fission reactors used in the United States for electric power production are classified as "light water reactors" in contrast to the "heavy water reactors" used in Canada. Light water (ordinary water) is used as the moderator in U.S. reactors as well as the cooling agent and the means by which heat is removed to produce steam for turning the turbines of the electric generators. The use of ordinary water makes it necessary to do a certain amount of enrichment of the uranium fuel before the necessary criticality of the reactor can be maintained.
 |
Uranium Enrichment
Natural uranium is only 0.7% U-235, the fissionable isotope. The other 99.3% is U-238 which is not fissionable. The uranium is usually enriched to 2.5-3.5% U-235 for use in U.S. light water reactors, while the heavy water Canadian reactors typically use natural uranium. Even with the necessity of enrichment, it still takes only about 3 kg of natural uranium to supply the energy needs of one American for a year.
Uranium enrichment has historically been accomplished by making the compound uranium hexaflouride and diffusing it through a long pathway of porous material (like kilometers!) and making use of the slightly higher diffusion rate of the lighter U-235 compound. There have been tests of centrifugal separators, but modern efforts are directed toward laser enrichment procedures.
The uranium fuel for fission reactors will not make a bomb; it takes enrichment to over 90% to obtain the fast chain reaction necessary for weapons applications. Enrichment to 15-30% is typical for breeder reactor.
Uranium Diffusion Enrichment
To produce the highly enriched uranium-235 needed for the development of nuclear weapons, a huge diffusion plant was built during World War II at Oak Ridge, Tennessee. Two other massive plants for uranium enrichment were built at Paducah, KY and Portsmout, OH after the war.
The compound uranium hexafluoride was produced and allowed to diffuse through thousands of stages of porous material, making use of the fact that the slightly lighter U-235 compound would diffuse faster than the U-238 compound. While electric power reactors require only enrichment from the 0.7% of natural uranium ore to about 3% U-235, the weapons applications required enrichment to over 90% U-235. Part of the enriched uranium was used to breed plutonium-239 for the more widely used plutonium devices.
Heavy Water Reactors
Nuclear fission reactors used in Canada use heavy water as the moderator in their reactors. Since the deuterium in heavy water is slightly more effective in slowing down the neutrons from the fission reactions, the uranium fuel needs no enrichment and can be used as mined. The Canadian style reactors are commonly called CANDU reactors.
Heavy water (D2O) is 10% heavier than ordinary water and has a neutron moderating ratio 80 times higher than ordinary water. As of January 2002, 32 of the 438 nuclear reactors in operation around the world were of CANDU type.
Nature's Nuclear Fission Reactor
In what is now Gabon in west Africa in 1972, French researchers found a deposit of uranium which had only 0.44% U-235 compared to the normal 0.72%. This indicated that some of the U-235 had undergone spontaneous nuclear fission at some point in the past. Also, fission-produced isotopes of neodynium and samarium were found. Some samples were found with a U-235 concentration as low as 0.29%. Models of the process suggested sustained fission reactions over a period of about a million years during a time period about two billion years ago.
The age estimate from cores in the reactor zones suggest a time frame between 1.7 and 1.9 billion years ago. For U-235 (halflife 700 million years) and U-238 (halflife 4.5 billion years), this would give a concentration of about 3% for the U-235 at the time of the reaction. It is presumed that ground water seeping through the ore served as a natural moderator to slow down the fission neutrons. One of the interesting observations was that the bulk of the fission products seemed to be still in place in their geologic depository after nearly 2 billion years. This could be taken as a suggestion that geologic storage of radioactive waste is feasible.
The fission of a U-235 nucleus in one fuel rod releases an average of 2.4 fast neutrons per fission. These neutrons are slowed down or "moderated" by the water between fuel rods, increasing the cross-section for neutron capture and fission by a U-235 nucleus in a neighboring fuel rod.
The two varieties of the light water reactor are the pressurized water reactor (PWR) and boiling water reactor (BWR).
Energy a la Einstein
Mass can be converted into energy with a yield governed by the Einstein relationship:
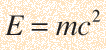
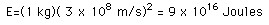
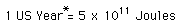 |
So one kilogram of mass conversion could supply the needs of about 180,000 U.S. citizens for one year, or the needs of a city of one million for over two months.
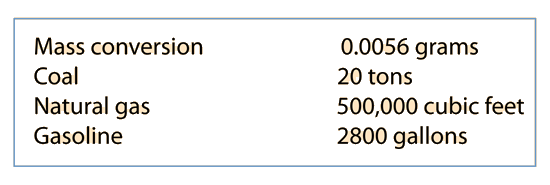
How Much Fuel per U.S. Citizen?
Nuclear Fission Reactors
Current uses of nuclear energy must rely on nuclear fission, a less-than-ideal energy source, since nuclear fusion has yet to be harnessed for electricity generation. The heat from the nuclear fission is used to:
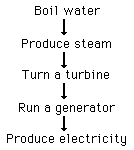

This usually done in a Boiling Water Reactor (BWR) or a Pressurized Water Reactor (PWR), but there are other options such as the fast breeder reactor.
Nuclear Electricity Generation
At the end of 1989 there were 416 nuclear fission power plants operating worldwide, producing about 17% of the worlds electricity. 130 more were in design or construction stages. The U.S. nuclear energy program is the world's largest with 108 operating plants with 100, 000 MW capacity, providing some 20% of the country's electricity in 1989. Coal provides about 55% of the country's electricity, natural gas (9%), oil (6%), and hydropower (9%).
Nuclear electricity generation became unpopular in the United States after the accident at Three Mile Island in 1979. No new orders for nuclear power plants have been placed in the US since the mid 1970s.
The three Mile Island Nuclear Plant disaster
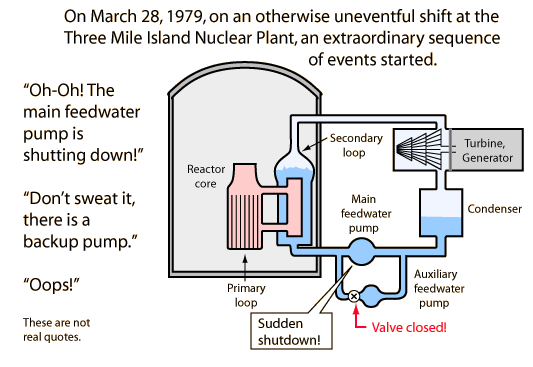
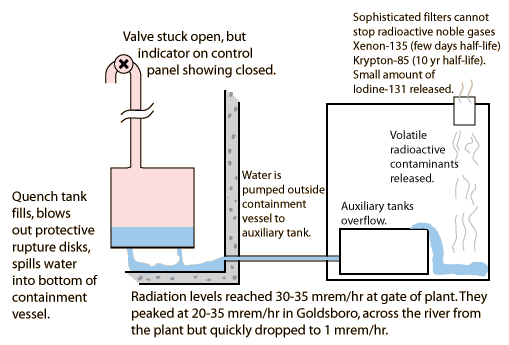
Misleading behavior of a safety valve quickly compounds the problem.
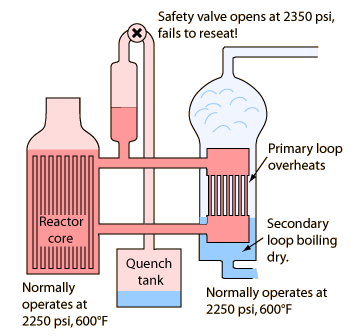
1. Secondary loop boils down to a low level, superheating the primary loop.
2. Pressure rises above 2350 psi, opening safety valve to quench tank. Radioactive steam and water move to quench tank.
3. Valve tries to close when pressure drops below 2300 psi, but doesn't seat. However, it closes enough to trip the indicator in the control room, so operators think it is closed.
|
The stuck relief valve leads to some radiation release
Meanwhile, back at the reactor ...
 |
- With the cooling effect of the restored feedwater and the stuck relief valve, the reactor pressure drops quickly.
- At 1600 psi the Emergency Core Cooling System kicks in automatically, flooding the core with high pressure water.
- Even though the reactor has "scrammed" and the control rods have stopped the fission chain reaction, the residual reactions still produce 5-7% of full heating power. For an 800 MW electrical, 2400 MW thermal reactor, this amounts to 120-170 Megawatts of heating power.
|
Three Mile Island: A Brief Chronology
| Time | Event |
| 0 | Condensate pump shuts down due to improperly closed valve in demineralizer. |
| 2 sec | Turbine automatically switches off. Pressure rises to 2255 psi, opens relief valve. It fails to close. |
| 8 sec | Sensing high pressure, reactor "scrams", dropping all control rods. |
| 9 sec | Fission reaction stops. (Fission products still produce heat.) |
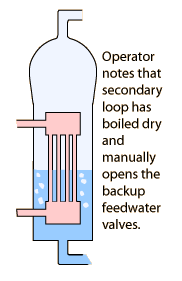
| 14 s | Operator notes that emergency feedwater pumps are on. |
| 1:45 | Steam generator (secondary loop) boils dry. |
| 2 min | Emergency core cooling system (ECCS) automatically turns on when pressure drops below 1600 psi. |
| 2.5 min | Operators shut down the ECCS!! |
The ECCS was shut off!!
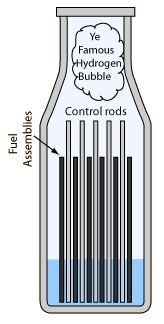 |
- The operators were afraid that the water level would get too high, that the reactor would "go solid". If the water filled the reactor, it would act like a hydraulic press and force the reactor lid off.
- After over an hour of heating, the coolant pumps were shut off, worsening the already bad situation.
- Two-thirds or more of the 12 foot core was uncovered. At 150 MW of heating it would take only 30 seconds for fuel melting to start if all cooling was lost.
- A large hydrogen "bubble" was formed at the top of the reactor by reaction between the zirconium fuel rod cladding and the superheated water.
The core was recovered with water after about 6.5 hours of exposure!!
|
| By this time the operators were dealing with about 100 alarms!! |


Sem comentários:
Enviar um comentário