Laser devices
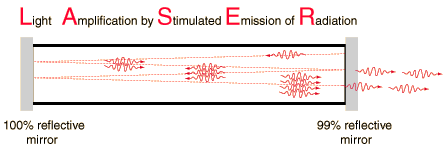
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation. Lasers differ from other sources of light because they emit light coherently. Spatial coherence allows a laser to be focused to a tight spot, enabling applications like laser cutting and lithography.
 |
Spatial coherence also allows a laser beam to stay narrow over long distances (collimation), enabling applications such as laser pointers. Lasers can also have high temporal coherence which allows them to have a very narrow spectrum, i.e., they only emit a single color of light. Temporal coherence can be used to produce pulses of light—as short as a femtosecond.
Lasers have many important applications. They are used in common consumer devices such as DVD players, laser printers, and barcode scanners. They are used in medicine for laser surgery and various skin treatments, and in industry for cutting and welding materials. They are used in military and law enforcement devices for marking targets and measuring range and speed.Laser lighting displays use laser light as an entertainment medium. Lasers also have many important applications in scientific research.
Quantum Properties of Light
Quantum processes dominate the fields of atomic and molecular physics. The treatment here is limited to a review of the characteristics of absorption, emission, and stimulated emission which are essential to an understanding of lasers and their applications.
Atomic transitions which emit or absorb visible light are generally electronic transitions, which can be pictured in terms of electron jumps between quantized atomic energy levels.
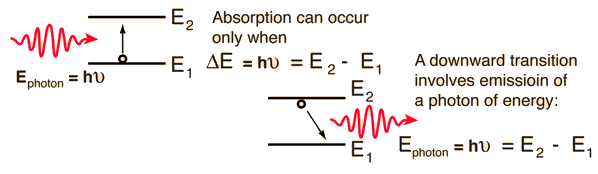
Note that the frequency that is emitted when an electron makes the downward transition is the same as the frequency absorbed by this two-level system. This can be generalized to the multiple energy levels of atoms. The emission spectra of atoms are the series of frequencies emitted by those atoms in gaseous form. If these same gases were cool, the same series of frequencies would be selectively absorbed.
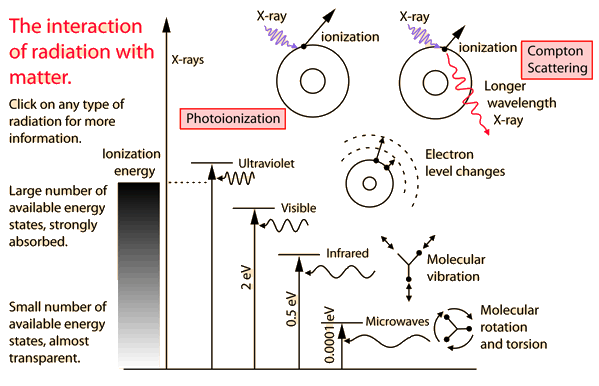
You may click on any of the types of radiation for more detail about its particular type of interaction with matter. The different parts of the electromagnetic spectrum have very different effects upon interaction with matter. Starting with low frequency radio waves, the human body is quite transparent. (You can listen to your portable radio inside your home since the waves pass freely through the walls of your house and even through the person beside you!)
As you move upward through microwaves and infrared to visible light, you absorb more and more strongly. In the lower ultraviolet range, all the uv from the sun is absorbed in a thin outer layer of your skin. As you move further up into the x-ray region of the spectrum, you become transparent again, because most of the mechanisms for absorption are gone. You then absorb only a small fraction of the radiation, but that absorption involves the more violent ionization events.
Each portion of the electromagnetic spectrum has quantum energies appropriate for the excitation of certain types of physical processes. The energy levels for all physical processes at the atomic and molecular levels are quantized, and if there are no available quantized energy levels with spacings which match the quantum energy of the incident radiation, then the material will be transparent to that radiation, and it will pass through.
Microwave Interactions
The quantum energy of microwave photons is in the range 0.00001 to 0.001 eV which is in the range of energies separating the quantum states of molecular rotation and torsion. The interaction of microwaves with matter other than metallic conductors will be to rotate molecules and produce heat as result of that molecular motion.
Conductors will strongly absorb microwaves and any lower frequencies because they will cause electric currents which will heat the material. Most matter, including the human body, is largely transparent to microwaves. High intensity microwaves, as in a microwave oven where they pass back and forth through the food millions of times, will heat the material by producing molecular rotations and torsions. Since the quantum energies are a million times lower than those of x-rays, they cannot produce ionization and the characteristic types of radiation damage associated with ionizing radiation.

Infrared Interactions
The quantum energy of infrared photons is in the range 0.001 to 1.7 eV which is in the range of energies separating the quantum states of molecular vibrations. Infrared is absorbed more strongly than microwaves, but less strongly than visible light. The result of infrared absorption is heating of the tissue since it increases molecular vibrational activity. Infrared radiation does penetrate the skin further than visible light and can thus be used for photographic imaging of subcutaneous blood vessels.

Visible Light Interactions
The primary mechanism for the absorption of visible light photons is the elevation of electrons to higher energy levels. There are many available states, so visible light is absorbed strongly. With a strong light source, red light can be transmitted through the hand or a fold of skin, showing that the red end of the spectrum is not absorbed as strongly as the violet end.
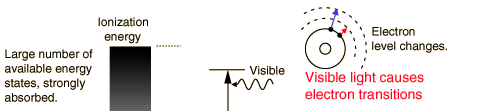
While exposure to visible light causes heating, it does not cause ionization with its risks. You may be heated by the sun through a car windshield, but you will not be sunburned - that is an effect of the higher frequency uv part of sunlight which is blocked by the glass of the windshield.
Lasers - Light Amplification Stimulated Emission Radiation
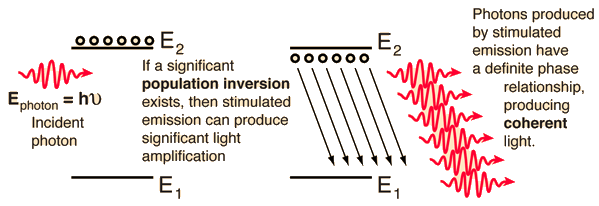
 | The achievement of a significant population inversion in atomic or molecular energy states is a precondition for laser action. Electrons will normally reside in the lowest available energy state. They can be elevated to excited states by absorption, but no significant collection of electrons can be accumulated by absorption alone since both spontaneous emission and stimulated emission will bring them back down. |
A population inversion cannot be achieved with just two levels because the probabability for absorption and for spontaneous emission is exactly the same, as shown by Einstein and expressed in the Einstein A and B coefficients. The lifetime of a typical excited state is about 10-8 seconds, so in practical terms, the electrons drop back down by photon emission about as fast as you can pump them up to the upper level. The case of the helium-neon laser illustrates one of the ways of achieving the necessary population inversion.
Parallel Light from a Laser
The light from a typical laser emerges in an extremely thin beam with very little divergence. Another way of saying this is that the beam is highly "collimated". An ordinary laboratory helium-neon laser can be swept around the room and the red spot on the back wall seems about the same size at that on a nearby wall.

The high degree of collimation arises from the fact that the cavity of the laser has very nearly parallel front and back mirrors which constrain the final laser beam to a path which is perpendicular to those mirrors. The back mirror is made almost perfectly reflecting while the front mirror is about 99% reflecting, letting out about 1% of the beam. This 1% is the output beam which you see. But the light has passed back and forth between the mirrors many times in order to gain intensity by the stimulated emission of more photons at the same wavelength. If the light is the slightest bit off axis, it will be lost from the beam.
The highly collimated nature of the laser beam contributes both to its danger and to its usefulness. You should never look directly into a laser beam, because the highly parallel beams can focus to an almost microscopic dot on the retina of your eye, causing almost instant damage to the retina. On the other hand, this capacity for sharp focusing contributes to the both the medical applications and the industrial applications of the laser. In medicine it is used as a sharp scalpel and in industry as a fast, powerful and computer-controllable cutting tool.
Monochromatic Laser Light
The light from a laser typically comes from one atomic transition with a single precise wavelength. So the laser light has a single spectral color and is almost the purest monochromatic light available.
That being said, however, the laser light is not exactly monochromatic. The spectral emission line from which it originates does have a finite width, if only from the Doppler effect of the moving atoms or molecules from which it comes. Since the wavelength of the light is extremely small compared to the size of the laser cavities used, then within that tiny spectral bandwidth of the emission lines are many resonant modes of the laser cavity.
Characteristics of Laser Light
1. Coherent. Different parts of the laser beam are related to each other in phase. These phase relationships are maintained over long enough time so that interference effects may be seen or recorded photographically. This coherence property is what makes holograms possible.
2. Monochromatic. Laser light consists of essentially one wavelength, having its origin in stimulated emission from one set of atomic energy levels.
3. Collimated. Because of bouncing back between mirrored ends of a laser cavity, those paths which sustain amplification must pass between the mirrors many times and be very nearly perpendicular to the mirrors. As a result, laser beams are very narrow and do not spread very much.
Helium-Neon Laser
The most common and inexpensive gas laser, the helium-neon laser is usually constructed to operate in the red at 632.8 nm. It can also be constructed to produce laser action in the green at 543.5 nm and in the infrared at 1523 nm.

The collimation of the beam is accomplished by mirrors on each end of the evacuated glass tube which contains about 85% helium and 15% neon gas at 1/300 atmospheres pressure (Metrologic). These mirrors could be both flat, but this requires great precision in alignment, so the common laboratory He-Ne lasers are manufactured with the semiconfocal mirror arrangement shown.
The helium gas in the laser tube provides the pumping medium to attain the necessary population inversion for laser action.
Free-Electron Laser
| The radiation from a free-electron laser is produced from free electrons which are forced to oscillate in a regular fashion by an applied field. They are therefore more like synchrotron light sources or microwave tubes than like other lasers. They are able to produce highly coherent, collimated radiation over a wide range of frequencies. The magnetic field arrangement which produces the alternating field is commonly called a "wiggler" magnet. | 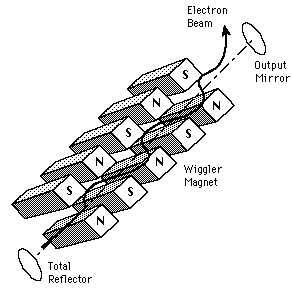 |
The free-electron laser is a highly tunable device which has been used to generate coherent radiation from 10^-5 to 1 cm in wavelength. In some parts of this range, they are the highest power source. Particularly in the mm wave range, the FELs exceed all other sources in coherent power. FELs involve relativistic electron beams propagating in a vacuum and can be tuned continuously, filling in frequency ranges which are not reachable by other coherent sources.
Applications of free-electron lasers are envisioned in isotope separation, plasma heating for nuclear fusion, long-range, high resolution radar, and particle acceleration in accelerators.
Dye Lasers
Tunable laser operation over a nearly continuous range of frequencies has been attained with the molecules of certain organic dyes. The molecules of these dyes have a large number of spectral lines and each of them has a characteristic spread of frequencies which is large compared to the spread of gaseous atomic spectral lines. With the overlap of these lines in the dyes, the dye laser can be tuned to produce laser action for laser spectroscopy.
A widely used dye is rhodamine 6G, commonly referred to as Rh6G. It is one of the most highly fluorescing materials known and was used by early astronauts to mark the position of their capsules when landing in the ocean. The unique properties which have made it useful in such exotic applications have also made it popular as a laser medium. Another dye used for spectroscopy is known as "ring dye" and is capable of essentially continuous tuning.
The dye laser medium is typically in liquid form and the dye is circulated continuously through the laser chamber to keep it from being limited by saturation effects. The dye may be pumped by flash lamps or by another laser such as an argon ion laser.
Laser Spectroscopy
Absorption spectroscopy usually implies having a tunable frequency source and producing a plot of absorption as a function of frequency. This was not feasible with lasers until the advent of the dye lasers which can be tuned over a nearly continuous range of frequencies.
Laser spectroscopy has led to advances in the precision with which spectral line frequencies can be measured, and this has fundamental significance for our understanding of basic atomic processes. This precision has been obtained by passing two laser beams through the absorption sample in opposite directions, selectively triggering absorption only in those atoms that have a zero velocity component in the direction of the beams. This effectively eliminates the Doppler broadening of spectral lines from the distribution of atomic velocities present in the sample.
| This work is in progress and will be posted when it's a little more develped. For a start, see laser oscillator. |  |
The Lamb Shift by Saturation Spectroscopy
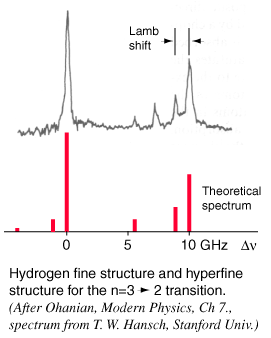
When you try to obtain very high resolution to examine small splittings in spectral lines, such as the hydrogen fine structure and the Lamb shift, those details are obscured by the sources of line broadening.
In a low pressure gas, the main source of broadening is Doppler broadening from the thermal motion of the atoms or molecules of the gas. This is particularly serious in hydrogen, since it has a low mass and therefore high thermal velocity.
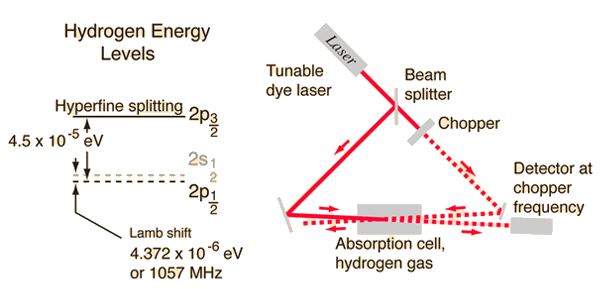
Tunable dye lasers are used to excellent advantage in "Doppler-free saturation spectroscopy" to minimize the effects of the Doppler broadening. The light from the laser is split into two beams, a saturating beam and a probe beam, arranged so that they cross in a region of a gas cell containing hydrogen gas.
When the laser is tuned to a the frequency of an electron transition, the saturating beam is intense enough to deplete the lower level of the transition. The saturating beam is "chopped", or modulated, so that the saturation of the transiton is turned on an off.
The probe beam is then absorbed, or not, depending on the whether the saturating beam is on. The probe beam strikes a sensitive detector, locked to the modulation frequency, which can then detect a signal at that frequency corresponding to the turning on and off of the absorption of the probe beam.
The probe beam is then absorbed, or not, depending on the whether the saturating beam is on. The probe beam strikes a sensitive detector, locked to the modulation frequency, which can then detect a signal at that frequency corresponding to the turning on and off of the absorption of the probe beam.
The tremendous advantage of this technique for eliminating the effects of Doppler broadening comes from directing the saturation beam and probe beam through the gas in opposite directions. The only hydrogen atoms which are in resonance with both of the beams are those which have no component of velocity in the direction of the beams, and therefore absorb at the frequency associated with the rest frame of the atom.
Other atoms in their rest frames see the incoming radiation shifted up for the saturation beam and down for probe beam or vice versa, so they are not resonant for both. The spectrum shown was obtained by tuning the dye laser slowly through the frequency range of the transition and measuring the change in intensity of the probe beam at the modulation or "chopping" frequency.
For hydrogen gas at 300 K, the rms velocity is about 2700 m/s. This speed corresponds to a Doppler shift of about 4 GHz, or a line broadening of twice that. This would effectively obscure the Lamb shift, since it is only 1.057 GHz.
The Lamb Shift
According to the hydrogen Shrodinger equation solution, the energy levels of the hydrogen electron should depend only on the principal quantum number n. In 1951, Willis Lamb discovered that this was not so - that the 2p(1/2) state is slightly lower than the 2s(1/2) state resulting in a slight shift of the corresponding spectral line (the Lamb shift).
| It might seem that such a tiny effect would be deemed insignificant, but in this case that shift probed the depths of our understanding of electromagnetic theory. |
This "smears out" the electron position over a range of about 0.1 fermi (Bohr radius = 52,900 fermis). This causes the electron spin g-factor to be slightly different from 2. There is also a slight weakening of the force on the electron when it is very close to the nucleus, causing the 2s electron (which has penetration all the way to the nucleus) to be slightly higher in energy than the 2p(1/2) electron.
| When we say the that the penetration of the 2s electron closer to the nucleus leads to an energy higher than that of the 2p, this sounds directly contradictory to the situation with multi-electron atoms. There the penetration of the 2s closer to the nucleus means that it has penetrated inside the 1s electrons and therefore feels a stronger attraction to the positive nucleus, leading to a lower energy level (it takes more energy to remove the 2s from the atom than the 2p). But in the case of the hydrogen atom, there is only one electron, so there is none of the shielding from inner electrons when it is in the 2s or 2p excited states. The effect on the energy levels has an entirely different origin, modeled by quantum electrodynamics. In the absence of this effect, the 2s and 2p would have identical energies since there is no shielding by the presence of other electrons. The "self-interaction" of the electron when it is near the proton causes the effective "smearing" of the electron charge so that its attraction to the proton is slightly weaker than it otherwise would have been. This means it has encountered an interaction which makes it slightly less tightly bound than a 2p electron, hence higher in energy. |
Significance of the Lamb Shift
When the Lamb shift was experimentally determined, it provided a high precision verification of theoretical calculations made with the quantum theory of electrodynamics. These calculations predicted that electrons continually exchanged photons, this being the mechanism by which the electromagnetic force acted. The effect of the continuous emission and absorption of photons on the electron g-factor could be calculated with great precision.
The tiny Lamb shift, measured with great precision, agreed to many decimal places with the calculated result from quantum electrodynamics. The measured precision gives us the electron spin g-factor as
Helium-Neon Laser
The most common and inexpensive gas laser, the helium-neon laser is usually constructed to operate in the red at 632.8 nm. It can also be constructed to produce laser action in the green at 543.5 nm and in the infrared at 1523 nm.
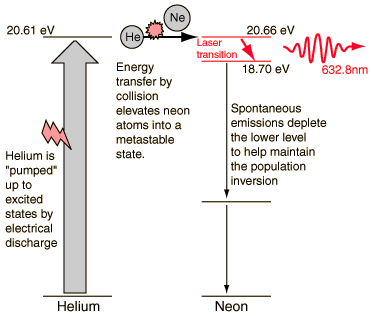  |
One of the excited levels of helium at 20.61 eV is very close to a level in neon at 20.66 eV, so close in fact that upon collision of a helium and a neon atom, the energy can be transferred from the helium to the neon atom.
Helium-neon lasers are common in the introductory physics laboratories, but they can still be dangerous! According to Garmire, an unfocused 1-mW HeNe laser has a brightness equal to sunshine on a clear day (0.1 watt/cm2) and is just as dangerous to stare at directly.
|
The helium gas in the laser tube provides the pumping medium to attain the necessary population inversion for laser action.

Carbon Dioxide Laser
The carbon dioxide gas laser is capable of continuous output powers above 10 kilowatts. It is also capable of extremely high power pulse operatin. It exhibits laser action at several infrared frequencies but none in the visible. Operating in a manner similar to the helium-neon laser, it employs an electric discharge for pumping, using a percentage of nitrogen gas as a pumping gas.
The CO2 laser is the most efficient laser, capable of operating at more than 30% efficiency. That's a lot more efficient than an ordinary incandescent light bulb at producing visible light (about 90% of the output of a lightbulb filament is invisible).
The carbon dioxide laser finds many applications in industry, particularly for welding and cutting.
Argon Laser
The argon ion laser can be operated as a continuous gas laser at about 25 different wavelengths in the visible between 408.9 and 686.1nm, but is best known for its most efficient transitions in the green at 488 nm and 514.5 nm. Operating at much higher powers than the helium-neon gas laser, it is not uncommon to achieve 30 to 100 watts of continuous power using several transitions. This output is produced in a hot plasma and takes extremely high power, typically 9 to 12 kW, so these are large and expensive devices.
Laser applications
Many scientific, military, medical and commercial laser applications have been developed since the invention of the laser in 1958. The coherency, high monochromaticity, and ability to reach extremely high powers are all properties which allow for these specialized applications.
Medical Uses of Lasers
The highly collimated beam of a laser can be further focused to a microscopic dot of extremely high energy density. This makes it useful as a cutting and cauterizing instrument. Lasers are used for photocoagulation of the retina to halt retinal hemorrhaging and for the tacking of retinal tears. Higher power lasers are used after cataract surgery if the supportive membrane surrounding the implanted lens becomes milky.
Photodisruption of the membrane often can cause it to draw back like a shade, almost instantly restoring vision. A focused laser can act as an extremely sharp scalpel for delicate surgery, cauterizing as it cuts. ("Cauterizing" refers to long-standing medical practices of using a hot instrument or a high frequency electrical probe to singe the tissue around an incision, sealing off tiny blood vessels to stop bleeding.) The cauterizing action is particularly important for surgical procedures in blood-rich tissue such as the liver.
Lasers have been used to make incisions half a micron wide, compared to about 80 microns for the diameter of a human hair.
Laser medicine
is the use of various types of lasers in medical diagnosis, treatment, or therapy. Types of lasers used in medicine include in principle any laser design, but especially:
- CO2 lasers
- diode lasers
- dye lasers
- excimer lasers
- fiber lasers
- gas lasers
- free electron lasers
- optical parametric oscillators
- angioplasty
- cancer diagnosis and treatment]
- cosmetic applications such as laser hair removal and tattoo removal
- dermatology]
- lithotripsy]
- mammography]
- medical imaging
- microscopy
- ophthalmology (includes Lasik and laser photo coagulation)
- optical coherence tomography
- prostatectomy
- surgery
Ophthalmology is the branch of medicine that deals with the anatomy, physiology and diseases of the eye. An ophthalmologist is a specialist in medical and surgical eye problems. Since ophthalmologists perform operations on eyes, they are both surgicaland medical specialists.
 The prominent opticians of the late 19th and early 20th century included Ernst Abbe (1840–1905), a co-owner of at the Zeiss Jena factories in Germany where he developed numerous optical instruments. Hermann von Helmholtz (1821-1894) was a polymath who made contributions to many fields of science and invented the ophthalmoscope in 1851. They both made theoretical calculations on image formation in optical systems and had also studied the optics of the eye.
The prominent opticians of the late 19th and early 20th century included Ernst Abbe (1840–1905), a co-owner of at the Zeiss Jena factories in Germany where he developed numerous optical instruments. Hermann von Helmholtz (1821-1894) was a polymath who made contributions to many fields of science and invented the ophthalmoscope in 1851. They both made theoretical calculations on image formation in optical systems and had also studied the optics of the eye.
An increased understanding of laser-tissue interactions in ophthalmology has led to the use of lasers in treating a wide spectrum of diseases involving both the anterior and posterior segments of the eye.
These diseases include the four commonest causes of blindness in the United States: diabetic retinopathy, age-related macular degeneration, glaucoma and cataract. As knowledge of the operating characteristics and treatment limitations of lasers increases, the use of lasers in the treatment of other ophthalmologic problems will be broadened.
Welding and Cutting
The highly collimated beam of a laser can be further focused to a microscopic dot of extremely high energy density for welding and cutting.
The automobile industry makes extensive use of carbon dioxide lasers with powers up to several kilowatts for computer controlled welding on auto assembly lines.
Garmire points out an interesting application of CO2 lasers to the welding of stainless steel handles on copper cooking pots.
A nearly impossible task for conventional welding because of the great difference in thermal conductivities between stainless steel and copper, it is done so quickly by the laser that the thermal conductivities are irrelevant.
A nearly impossible task for conventional welding because of the great difference in thermal conductivities between stainless steel and copper, it is done so quickly by the laser that the thermal conductivities are irrelevant.
Surveying and Ranging
Helium-neon and semiconductor lasers have become standard parts of the field surveyor's equipment. A fast laser pulse is sent to a corner reflector at the point to be measured and the time of reflection is measured to get the distance.
Some such surveying is long distance! The Apollo 11 and Apollo 14 astronauts put corner reflectors on the surface of the Moon for determination of the Earth-Moon distance. A powerful laser pulse from the MacDonald Observatory in Texas had spread to about a 3 km radius by the time it got to the Moon, but the reflection was strong enough to be detected. We now know the range from the Moon to Texas within about 15 cm, a nine significant digit measurement. A pulsed ruby laser was used for this measurement.
Lasers in the Garment Industry
Laser cutters are credited with keeping the U.S. garment industry competitive in the world market. Computer controlled laser garment cutters can be programmed to cut out 400 size 6 and then 700 size 9 garments - and that might involve just a few cuts. The programmed cutter can cut dozens to hundreds of thicknesses of cloth, and can cut out every piece of the garment in a single run.
The usefulness of the laser for such cutting operations comes from the fact that the beam is highly collimated and can be further focused to a microscopic dot of extremely high energy density for cutting.
Lasers in Communication
Fiber optic cables are a major mode of communication partly because multiple signals can be sent with high quality and low loss by light propagating along the fibers. The light signals can be modulated with the information to be sent by either light emitting diodes or lasers. The lasers have significant advantages because they are more nearly monochromatic and this allows the pulse shape to be maintained better over long distances. If a better pulse shape can be maintained, then the communication can be sent at higher rates without overlap of the pulses. Ohanian quotes a factor of 10 advantage for the laser modulators.
Telephone fiber drivers may be solid state lasers the size of a grain of sand and consume a power of only half a milliwatt. Yet they can sent 50 million pulses per second into an attached telephone fiber and encode over 600 simultaneous telephone conversations (Ohanian).
Heat Treatment
Heat treatments for hardening or annealing have been long practiced in metallurgy. But lasers offer some new possibilities for selective heat treatments of metal parts. For example, lasers can provide localized heat treatments such as the hardening of the surfaces of automobile camshafts. These shafts are manufactured to high precision, and if the entire camshaft is heat treated, some warping will inevitably occur. But the working surfaces of the cams can be heated quickly with a carbon dioxide laser and hardened without appreciably affecting the remainder of the shaft, preserving the precision of manufacture.
Barcode Scanners
Supermarket scanners typically use helium-neon lasers to scan the universal barcodes to identify products.
The laser beam bounces off a rotating mirror and scans the code, sending a modulated beam to a light detector and then to a computer which has the product information stored. Semiconductor lasers can also be used for this purpose.
Laser Fusion
Laser fusion attempts to force nuclear fusion in tiny pellets or microballoons of a deuterium-tritium mixture by zapping them with such a high energy density that they will fuse before they have time to move away from each other. This is an example of inertial confinement.
Two experimental laser fusion devices have been developed at Lawrence Livermore Laboratory, called Shiva and Nova. They deliver high power bursts of lase light from multiple lasers onto a small deuterium-tritium target. These lasers are neodymium glass lasers which are capable of extremely high power pulses.
Shiva Laser System
During the Shiva project at Lawrence Livermore Laboratories, a collection of 20 neodynium lasers were focused to a precise position in a target chamber. The multi-laser device, called Shiva after the multi-armed Hindu god, sought to initiate laser fusion in small microballoons of a deuterium-tritium gas mixture. One of the 0.1 mm pellets is supposed to contain the energy equivalent of a barrel of oil.
The Shiva system was the first generation machine at Livermore, put into operation in 1978. It was operated until 1981. A second, more powerful machine called Nova has been built which offers the possibility of reaching the fusion breakeven point.

Nova is the name given to the second generation laser fusion device at Lawrence Livermore Laboratories. It employs lasers ten times more powerful than the Shiva laser fusion device and will attempt to reach the breakeven point for fusion. Nova makes use of ten lasers which are focused on a 1 mm diameter target area, dumping 100,000 joules of energy into the target in a nanosecond. As of 1994, Nova has reached the Lawson criterion, but at a temperature too low for fusion ignition.

Particle Beam Fusion
If a high energy beam of electrons or other particles can be directed onto a tiny pellet or microballoon of deuterium-tritium mixture, it could cause it to explode like a miniature hydrogen bomb, fusing the deuterium and tritium nuclei in a time frame too short for them to move apart.
Laser Printers
The laser printer has in a few years become the dominant mode of printing in offices. It employs a semiconductor laser and the xerography principle. The laser is focused and scanned across a photoactive selenium coated drum where it produces a charge pattern which mirrors the material to be printed. This drum then holds the particles of the toner to transfer to paper which is rolled over the drum in the presence of heat. The typical laser for this application is the aluminum-gallium-arsenide (AlGaAs) laser at 760 nm wavelength, just into the infrared.
Laser Cooling
Starting in about 1985 with the work of Steven Chu and others, the use of lasers to achieve extremely low temperatures has advanced to the point that temperatures of 10-9 K have been reached. If an atom is traveling toward a laser beam and absorbs a photon from the laser, it will be slowed by the fact that the photon has momentum p = E/c = h/λ. If we take a sodium atom as an example, and assume that a number of sodium atoms are freely moving in a vacuum chamber at 300K, the rms velocity of a sodium atom from the Maxwell speed distribution would be about 570 m/s.
Then if a laser is tuned just below one of the sodium d-lines (589.0 and 589.6 nm, about 2.1 eV), a sodium atom traveling toward the laser and absorbing a laser photon would have its momentum reduced by the amount of the momentum of the photon. It would take a large number of such absorptions to cool the sodium atoms to near 0K since one absorption would slow a sodium atom by only about 3 cm/s out of a speed of 570 m/s. A straight projection requires almost 20,000 photons to reduce the sodium atom momentum to zero. The change in speed from the absorption of one photon can be calculated from
Δv = pphoton/m
That seems like a lot of photons, but according to Chu, a laser can induce on the order of 107 absorptions per second so that an atom could be stopped in a matter of milliseconds.
A conceptual problem is that an absorption can also speed up an atom if it catches it from behind, so it is necessary to have more absorptions from head-on photons if your goal is to slow down the atoms. This is accomplished in practice by tuning the laser slightly below the resonance absorption of a stationary sodium atom.
From the atom's perspective, the headon photon is seen as Doppler shifted upward toward its resonant frequency and it therefore more strongly absorbed than a photon traveling in the opposite direction which is Doppler shifted away from the resonance. In the case of our room temperature sodium atom above, the incoming photon would be Doppler shifted up 0.97 GHz, so to get the head on photon to match the resonant frequency would require that the laser be tuned below the resonant peak by that amount.
 This method of cooling sodium atoms was proposed by Theodore Hansch and Arthur Schawlow at Stanford University in 1975 and achieved by Chu at AT&T Bell Labs in 1985. Sodium atoms were cooled from a thermal beam at 500K to about 240 mK. The experimental technique involved directing laser beams from opposite directions upon the sample,linearly polarized at 90° with respect to each other. Six lasers could then provide a pair of beams along each coordinate axis. The effectively "viscous" effect of the laser beams in slowing down the atoms was dubbed "optical molasses" by Chu.
This method of cooling sodium atoms was proposed by Theodore Hansch and Arthur Schawlow at Stanford University in 1975 and achieved by Chu at AT&T Bell Labs in 1985. Sodium atoms were cooled from a thermal beam at 500K to about 240 mK. The experimental technique involved directing laser beams from opposite directions upon the sample,linearly polarized at 90° with respect to each other. Six lasers could then provide a pair of beams along each coordinate axis. The effectively "viscous" effect of the laser beams in slowing down the atoms was dubbed "optical molasses" by Chu.Continuing to cool the sodium atoms by this method requires the tuning of the laser upward in frequency toward the atomic resonant frequency because the Doppler shift will be smaller. This places a practical limit on how much cooling can be achieved, because the differential cooling rate is reduced and at a certain point the cooling mechanism is foiled by heating due to the random absorption and reemission of photons.
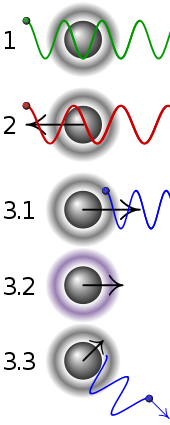
In the picture : Simplified principle of Doppler laser cooling:
| 1 | A stationary atom sees the laser neither red- nor blue-shifted and does not absorb the photon. |
|---|---|
| 2 | An atom moving away from the laser sees it red-shifted and does not absorb the photon. |
| 3.1 | An atom moving towards the laser sees it blue-shifted and absorbs the photon, slowing the atom. |
| 3.2 | The photon excites the atom, moving an electron to a higher quantum state. |
| 3.3 | The atom re-emits a photon. As its direction is random, there is no net change in momentum over many atoms. |
This practical limit is characterized by 2kT = Eresonant photon, which at the low temperature of 240 mK would correspond to photon energies around 4 x 10-8 eV. Such energies can characterize the Zeeman split energy levels of the atoms in the magnetic fields produced by the laser photons.
It was found that the splittings which limited the original laser cooling processes could be exploited to lower the ultimate temperatures below these limits.
With the opposing laser beams with perpendicular linear polarization, atoms could be selectively driven or "optically pumped" into the lower energy levels. These lasers create a small region of space about a quarter wavelength in extent where the atoms can drift to a region where their energy is relatively higher, only to be pumped downward to a lower energy again.
It was found that the splittings which limited the original laser cooling processes could be exploited to lower the ultimate temperatures below these limits.
With the opposing laser beams with perpendicular linear polarization, atoms could be selectively driven or "optically pumped" into the lower energy levels. These lasers create a small region of space about a quarter wavelength in extent where the atoms can drift to a region where their energy is relatively higher, only to be pumped downward to a lower energy again.
This was dubbed "Sisyphus cooling" after the legendary tormented man who was condemned to perpetually roll a rock up a hill, only to have it roll down again. With the "optical molasses" and the polarization gradient in the region of opposing laser beams, temperatures as low as 35 mK for sodium and 3 mK for cesium were obtained.
Cooling by Magnetic Trapping
Starting in about 1985 with the work of Steven Chu and others, the use of lasers to achieve extremely low temperatures has advanced to the point that temperatures of 10-9 K have been reached. The first big step toward the nanokelvin temperatures was laser cooling. With advances in technology and techniques colorfully dubbed "Sisyphus cooling" and "optical molasses", temperatures as low as 35 mK for sodium and 3 mK for cesium were obtained.
The laser cooling faced a hurdle known as the Doppler limit, which was partially overcome using magnetic fields in what was called a magneto-optical trap (MOT). During this period the quest for Bose-Einstein condensation in dilute gases was forming, and it needed yet lower temperatures.
Once a magnetic trap had been achieved, it was realized that the establishment of a "deep" trap and then the lowering of the walls of the trap would allow the most energetic of the atoms to escape, leaving a collection with lower energies and a lower temperature. This was described as cooling by evaporation, and it had the dual effect of lowering the temperature and raising the density of the remaining trapped atoms. Both of these effects helped toward the goal of establishing the Bose-Einstein Condensate (BEC).
Compact Disc
Audio Analog sound data is digitized by sampling at 44.1 kHz and coding as binary numbers in the pits on the compact disc. As the focused laser beam sweeps over the pits, it reproduces the binary numbers in the detection circuitry. The same function as the "pits" can be accomplished by magnetooptical recording. The digital signal is then reconverted to analog form by a D/A converter.
The tracks on a compact disc are nominally spaced by 1.6 micrometers, close enough that they are able to separate reflected light into it's component colors like a diffraction grating.
This is an active graphic.
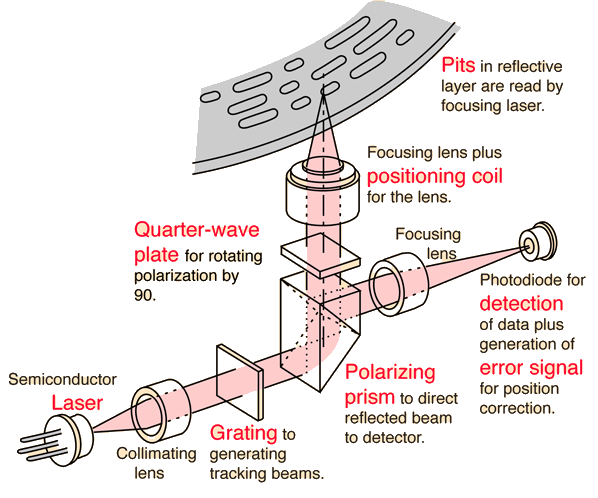
Laser for Compact Discs
The detection of the binary data stored in the form of pits on the compact disc is done with the use of a semiconductor laser. The laser is focused to a diameter of about 0.8 mm at the bottom of the disc, but is further focused to about 1.7 micrometers as it passes through the clear plastic substrate to strike the reflective layer.
The Philips CQL10 laser has a wavelength of 790 nm in air. The depth of the pits is about a quarter of the wavelength of this laser in the substrate material.

A polarizing prism is made up of two prisms of a birefringent material joined along a diagonal. The angle of cut is such that the plane of polarization parallel to the surface undergoes total internal reflection whereas the plane perpendicular to the surface passes through. Because of the action of the quarter-wave plate, the beam returning from the disc will be polarized parallel to the surface and will be reflected 90°, toward the photodiode detector. This is an active graphic. Click on any bold text for further details.

Laser light from the reflective layer of the disc returns through the quarter-wave plate. This causes it to reflect in the beam-splitter so that it reaches the photodiode for detection. However, if the beam strikes one of the pits, which are about a quarter- wavelength in depth, the light is out of phase with the light reflecting from the unaltered plane around it and tends to cancel it. This produces enough change in light level to be detected by the photodiode, and to be coded as the 0's and 1's of binary data. This is an active graphic. Click on any bold text for further details.

Laser Beam Positioning
In order to be reliably decoded, the laser beam must be focused within about 0.5 micrometers of the reflective surface, but the location of the bottom of the disc may be uncertain by about 0.5 mm during rotation. To keep the beam focused, a positioning coil drives the focusing lens up or down in response to an error voltage from the detector. One scheme uses a cylindrical lens arrangement to focus light on the detector. When the beam is properly focused, it projects a round beam and a zero error voltage results.

Microscopy
Confocal laser scanning microscopy and Two-photon excitation microscopy make use of lasers to obtain blur-free images of thick specimens at various depths. Laser capture microdissection use lasers to procure specific cell populations from a tissue section under microscopic visualization.
Additional laser microscopy techniques include harmonic microscopy, four-wave mixing microscopy and interferometric microscopy.
Nuclear fusion
Some of the world's most powerful and complex arrangements of multiple lasers and optical amplifiers are used to produce extremely high intensity pulses of light of extremely short duration. These pulses are arranged such that they impact pellets of tritium-deuterium simultaneously from all directions, hoping that the squeezing effect of the impacts will induce atomic fusion in the pellets. This technique, known as "inertial confinement fusion", so far has not been able to achieve "breakeven", that is, so far the fusion reaction generates less power than is used to power the lasers, but research continues.
Military applications
Military uses of lasers include applications such as target designation and ranging, defensive countermeasures, communications and directed energy weapons.
Target designator
Another military use of lasers is as a laser target designator. This is a low-power laser pointer used to indicate a target for a precision-guided munition, typically launched from an aircraft. The guided munition adjusts its flight-path to home in to the laser light reflected by the target, enabling a great precision in aiming.
The beam of the laser target designator is set to a pulse rate that matches that set on the guided munition to ensure munitions strike their designated targets and do not follow other laser beams which may be in use in the area.
The laser designator can be shone onto the target by an aircraft or nearby infantry.
Lasers used for this purpose are usually infrared lasers, so the enemy cannot easily detect the guiding laser light.
Laser sight
The laser has in most firearms applications been used as a tool to enhance the targeting of other weapon systems. For example, a laser sight is a small, usually visible-light laser placed on a handgun or a rifle and aligned to emit a beam parallel to the barrel.
Since a laser beam has low divergence, the laser light appears as a small spot even at long distances; the user places the spot on the desired target and the barrel of the gun is aligned (but not necessarily allowing for bullet drop, windage, distance between the direction of the beam and the axis of the barrel, and the target mobility while the bullet travels).
Most laser sights use a red laser diode. Others use an infrared diode to produce a dot invisible to the naked human eye but detectable with night vision devices.
The firearms adaptive target acquisition module LLM01 laser light module combines visible and infrared laser diodes. In the late 1990s, green diode pumped solid state laser (DPSS) laser sights (532 nm) became available. Modern laser sights are small and light enough for attachment to the firearms.
In 2007, LaserMax, a company specializing in manufacturing lasers for military and police firearms, introduced the first mass-production green laser available for small arms.
This laser mounts to the underside of a handgun or long arm on the accessory rail. The green laser is supposed to be more visible than the red laser in bright lighting conditions because, for the same wattage, green light appears brighter than red light.








ishq pakeezah mp3 download,mera ishq hai pakeezah mp3 song,ishq pakeezah ringtone download,punjabi gane video,ishq pakeezah video Online,mera ishq hai pakeezah mp3 song,ishq pakeezah lyrics,ishq pakeezah punjabi song,sad song vich.ishq pakeezah video of Indian Serials Online Free. Watch Full Episodes of Star Plus, colors TV, Zee TV, Sony TV.http://ishqpakeezah.com
ResponderEliminarIshq Pakeezah
Drama cool
Drama cool